Predictive value of composite inflammatory markers combined with T-cell cytokines for the prognosis of sepsis patients complicated with severe acute kidney injury
-
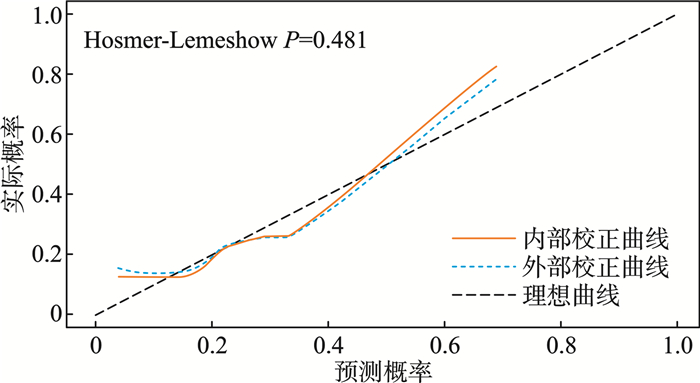
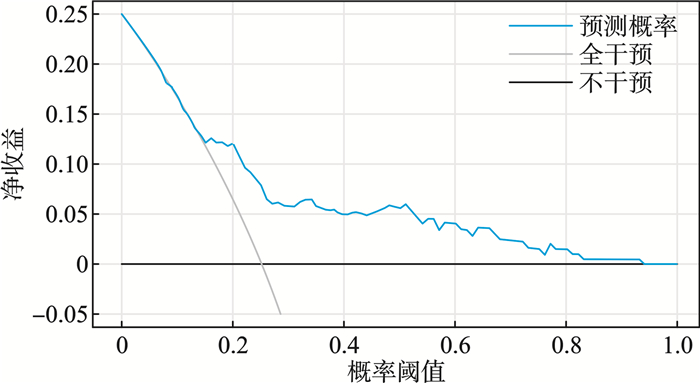
摘要: 目的 探讨合并严重急性肾损伤的脓毒症患者外周血复合炎性标志物及血清T细胞因子特征及联合预测患者预后的价值。方法 选取2022年6月—2024年7月在衡水市第二人民医院急诊科收治的合并严重急性肾损伤的脓毒症患者195例作为研究组,另外选择同期治疗的50例脓毒症患者作为单纯脓毒症组、行体检的50例健康人作为对照组。研究组患者根据28 d内是否死亡划分为死亡组(49例)和存活组(146例)。对比不同组患者间一般人口学资料、外周血复合炎性标志物[中性粒细胞/淋巴细胞比值(neutrophil-to-lymphocyte ratio,NLR)、血小板/淋巴细胞比值(platelet-to-lymphocyte ratio,PLR)、系统免疫炎症指数(systemic immune-inflammation index,SII)、全身炎症反应指数(systemic inflammatory response index,SIRI)]及血清T细胞因子[肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白细胞介素1(interleukin-1,IL-1)、IL-6、IL-17、IL-35]水平的差异。通过单因素、多因素logistic回归分析评价单因素分析中P<0.05的指标与合并严重急性肾损伤的脓毒症患者短期死亡的相关性;通过受试者工作特征曲线(receiver operating characteristic curve,ROC)、曲线下面积(area under the curve,AUC)评价各危险因素独立及联合预测合并严重急性肾损伤的脓毒症患者短期死亡的效能;通过校准曲线和决策曲线(decision curve analysis,DCA)评价联合预测模型的拟合度和净收益。结果 研究组患者NLR、PLR、SII、SIRI、TNF-α、IL-1、IL-6、IL-17、IL-35均显著高于单纯脓毒症组和对照组(P<0.05),死亡组患者SOFA评分、APACHEⅡ评分、SII、SIRI、IL-1、IL-17、IL-35均显著高于存活组(P<0.05);单因素及多因素logistic回归分析提示SII、SIRI、IL-1、IL-17、IL-35均与合并严重急性肾损伤的脓毒症患者短期死亡存在显著相关性(P<0.05);ROC曲线分析表明SII、SIRI、IL-1、IL-17、IL-35独立及联合预测合并严重急性肾损伤的脓毒症患者短期死亡风险的AUC均较高(P<0.05)。Delong检验表明联合预测AUC显著大于SII、SIRI、IL-1、IL-17、IL-35独立预测的AUC(Z=3.664、3.451、4.013、3.448、3.378,P<0.05)。校准曲线分析表明基于SII、SIRI、IL-1、IL-17、IL-35的联合预测模型拟合度较好(Hosmer-Lemeshow,P=0.481),DCA分析提示二者联合预测在阈值概率0~0.850均可提供最大净收益。结论 合并严重急性肾损伤的脓毒症患者SII、SIRI、IL-1、IL-17、IL-35水平较高均与短期死亡风险密切相关,基于上述指标的预测模型在辅助判断合并严重急性肾损伤的脓毒症患者短期死亡风险中具有较高价值。Abstract: Objective To explore the characteristics of peripheral blood composite inflammatory markers and serum T-cell cytokines in sepsis patients with severe acute kidney injury, and the value of their combined use in predicting the patients' prognosis.Methods From June 2022 to July 2024, 195 cases of patients with sepsis complicated by severe acute kidney injury admitted to Hengshui Second People's Hospital were selected as the study group. Additionally, 50 cases of patients with sepsis who received treatment during the same period were chosen as the pure sepsis group, and 50 healthy individuals who underwent physical examinations were selected as the control group. Among the patients in the study group, 49 cases belonged to the deceased group while 146 cases were in the survival group, based on their survival status within 28 days. The analysis encompassed the comparison of demographic characteristics, peripheral blood composite inflammatory markers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammatory index (SII), systemic inflammatory response index (SIRI), and serum T-cell factors, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, IL-17, IL-35, between different groups. Univariate and multivariate logistic regression analyses were carried out to investigate the association between indicators identified with a significance level of P < 0.05 in the univariate analysis and short-term mortality outcomes in patients with sepsis complicated by severe acute kidney injury. The predictive performance of each risk factor for short-term mortality was evaluated through receiver operating characteristic (ROC) curves and calculation of the area under the curve (AUC). Furthermore, the calibration curves and decision curve analysis (DCA) were utilized to assess the goodness-of-fit and net benefit of the combined prediction model.Results In the study group, the levels of NLR, PLR, SII, SIRI, TNF-α, IL-1, IL-6, IL-17, and IL-35 were found to be significantly elevated compared to those in the pure sepsis group and control group (P < 0.05). It was observed that the deceased group had significantly higher SOFA scores, APACHE Ⅱ scores, SII, SIRI, IL-1, IL-17, and IL-35 compared to the survival group (P < 0.05). Both univariate and multivariate logistic regression analyses showed strong associations between SII, SIRI, IL-1, IL-17, IL-35, and short-term mortality among sepsis patients with severe acute kidney injury (P < 0.05). The ROC curve analysis demonstrated high AUC values for SII, SIRI, IL-1, IL-17, and IL-35 in predicting the risk of short-term mortality in these patients (P < 0.05). Additionally, the combined prediction AUC obtained from the DeLong test was significantly greater than the AUCs of individual predictors (SII, SIRI, IL-1, IL-17, IL-35) (Z=3.664, 3.451, 4.013, 3.448, 3.378, P < 0.05). The calibration curve analysis indicated that the combined prediction model based on SII, SIRI, IL-1, IL-17, and IL-35 was well-fitted (Hosmer-Lemeshow, P=0.481), and the DCA analysis suggested that within a threshold probability range of 0 to 0.850, the combined prediction model provided the maximum net benefit.Conclusion Heightened concentrations of SII, SIRI, IL-1, IL-17, and IL-35 in individuals with sepsis complicated by severe acute kidney injury are strongly linked to the risk of short-term mortality. A predictive model incorporating these biomarkers demonstrated high clinical utility in assessing short-term mortality risk for these patients.
-
Key words:
- sepsis /
- acute kidney injury /
- inflammatory markers /
- cytokines /
- mortality risk
-

-
表 1 研究组、单纯脓毒症组和对照组的基本资料比较
X±S 指标 研究组(195例) 单纯脓毒症组(50例) 对照组(50例) F/χ2 P 年龄/岁 58.40±3.45 58.34±3.22 57.94±3.24 0.372 0.690 性别/例(%) 0.309 0.857 男 106(54.4) 25(50.0) 27(54.0) 女 89(45.6) 25(50.0) 23(46.0) BMI/(kg/m2) 23.28±1.31 23.35±1.31 22.94±1.13 1.642 0.195 吸烟史/例(%) 0.037 0.982 有 92(47.2) 23(46.0) 23(46.0) 无 103(52.8) 27(54.0) 27(54.0) 饮酒史/例(%) 0.195 0.907 有 76(39.0) 20(40.0) 18(36.0) 无 119(61.0) 30(60.0) 32(64.0) 基础疾病史/例(%) 1.682 0.947 高血压 40(20.5) 12(24.0) 11(22.0) 糖尿病 33(16.9) 9(18.0) 10(20.0) 冠心病 41(21.1) 10(20.0) 7(14.0) 无 81(41.5) 19(38.0) 22(44.0) 表 2 死亡组和存活组的基本资料比较
X±S 指标 死亡组(49例) 存活组(146例) t/χ2 P 年龄/岁 58.59±3.37 58.34±3.48 0.449 0.654 性别/例(%) 0.015 0.904 男 27(55.1) 79(54.1) 女 22(44.9) 67(45.9) BMI/(kg/m2) 23.23±1.47 23.29±1.26 0.249 0.804 吸烟史/例(%) 0.085 0.771 有 24(49.0) 68(46.6) 无 25(51.0) 78(53.4) 饮酒史/例(%) 0.001 0.974 有 19(38.8) 57(39.0) 无 30(61.2) 89(61.0) 基础疾病史/例(%) 1.449 0.484 高血压 18(36.7) 62(46.6) 糖尿病 18(36.7) 46(31.5) 冠心病 13(26.5) 32(21.9) SOFA评分/分 8.88±2.25 8.12±2.16 2.094 0.038 APACHEⅡ/分 23.80±3.07 22.94±2.10 2.187 0.030 表 3 研究组、单纯脓毒症组和对照组间外周血复合炎性标志物及血清T细胞因子比较
X±S 观察指标 研究组(195例) 单纯脓毒症组(50例) 对照组(50例) F P NLR 11.03±1.67 9.43±1.36 1.80±0.13 782.889 <0.001 PLR 286.67±56.40 245.33±46.17 97.80±3.78 277.948 <0.001 SII 1 236.40±208.50 1 084.01±151.62 287.35±43.41 543.240 <0.001 SIRI 7.17±1.68 6.11±1.39 1.29±0.27 310.967 <0.001 TNF-α/(pg/mL) 138.77±21.80 126.46±20.96 10.14±2.05 853.962 <0.001 IL-1/(pg/mL) 134.31±20.99 116.20±18.14 9.26±2.13 895.631 <0.001 IL-6/(pg/mL) 112.20±15.63 101.00±15.18 13.38±3.03 968.307 <0.001 IL-17/(pg/mL) 79.21±16.00 70.13±13.83 7.41±1.34 509.858 <0.001 IL-35/(pg/mL) 149.21±24.60 133.51±20.20 10.82±1.95 815.910 <0.001 表 4 死亡组和存活组间外周血复合炎性标志物及血清T细胞因子比较
X±S 指标 死亡组(49例) 存活组(146例) t P NLR 11.12±1.78 11.00±1.64 0.453 0.651 PLR 288.68±64.94 281.99±53.37 0.718 0.474 SII 1307.21±312.51 1212.64±153.53 2.795 0.006 SIRI 7.76±2.19 6.96±1.42 2.944 0.004 TNF-α/(pg/mL) 137.58±24.41 139.17±20.93 0.443 0.658 IL-1/(pg/mL) 141.13±23.63 132.02±19.59 2.671 0.008 IL-6/(pg/mL) 111.12±15.43 112.57±15.73 0.558 0.578 IL-17/(pg/mL) 84.15±16.49 77.55±15.54 2.533 0.012 IL-35/(pg/mL) 157.24±27.15 146.52±23.15 2.682 0.008 表 5 合并严重急性肾损伤的脓毒症患者短期死亡风险的危险因素单因素logistic回归分析
指标 β SE Wald χ2 P OR(95%CI) SOFA评分 0.160 0.078 2.057 0.040 1.174(1.008~1.367) APACHEⅡ评分 0.149 0.070 2.144 0.032 1.161(1.013~1.330) SII 0.002 0.001 2.700 0.007 1.002(1.001~1.004) SIRI 0.288 0.102 2.825 0.005 1.334(1.092~1.629) IL-1 0.022 0.008 2.587 0.010 1.022(1.005~1.039) IL-17 0.026 0.011 2.466 0.014 1.027(1.005~1.048) IL-35 0.018 0.007 2.597 0.009 1.019(1.005~1.033) 表 6 合并严重急性肾损伤的脓毒症患者短期死亡风险的危险因素多因素logistic回归分析
指标 β SE Wald χ2 P OR(95%CI) 常量 -18.938 3.441 -5.503 <0.001 0 SOFA评分 0.023 0.012 1.924 0.054 1.024(1.000~1.048) APACHEⅡ评分 0.002 0.001 1.725 0.085 1.002(1.000~1.003) SII 0.161 0.079 2.044 0.041 1.175(1.007~1.371) SIRI 0.287 0.118 2.441 0.015 1.333(1.058~1.679) IL-1 0.022 0.009 2.403 0.016 1.022(1.004~1.041) IL-17 0.207 0.090 2.298 0.022 1.230(1.031~1.467) IL-35 0.023 0.008 2.750 0.006 1.023(1.007~1.039) 表 7 合并严重急性肾损伤的脓毒症患者短期死亡风险的危险因素多因素logistic回归分析
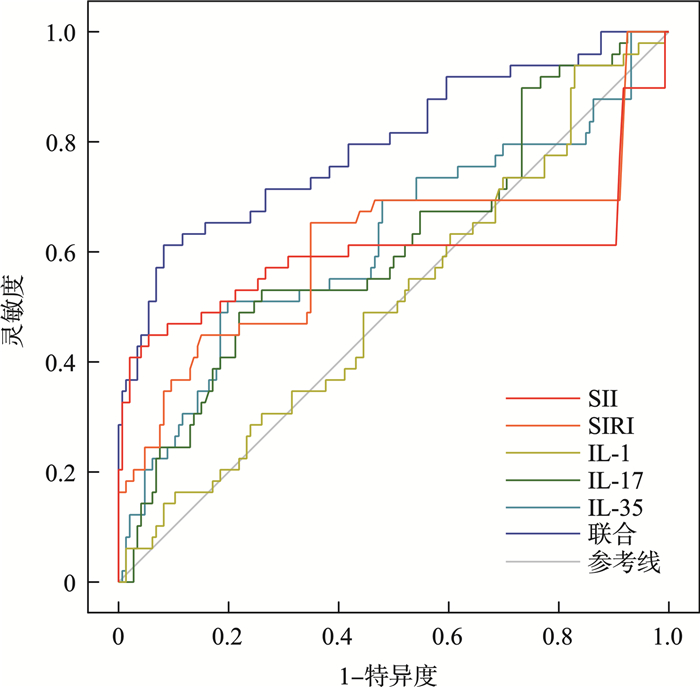
危险因素 AUC SE 95%CI P 截断值 灵敏度/% 特异度/% 约登指数 SII 0.596 0.062 0.474~0.718 0.045 1 458.13 44.90 94.52 0.394 SIRI 0.608 0.056 0.499~0.717 0.024 7.58 65.31 65.07 0.304 IL-1 0.512 0.048 0.418~0.606 0.026 127.23 pg/mL 93.88 17.12 0.110 IL-17 0.612 0.048 0.517~0.707 0.019 87.79 pg/mL 48.98 78.08 0.271 IL-35 0.620 0.050 0.521~0.719 0.012 162.61 pg/mL 51.02 80.14 0.312 联合 0.796 0.041 0.716~0.876 <0.001 - 61.22 91.78 0.530 -
[1] 罗仁杰. 脓毒症相关急性肾损伤患者的危险因素及预后分析[D]. 重庆医科大学, 2021.
[2] 王志勇, 王刚, 才仔全. 脓毒症并发急性肾损伤患者临床特征及预后的影响因素[J]. 临床急诊杂志, 2022, 23(8): 566-571. doi: 10.13201/j.issn.1009-5918.2022.08.005
[3] 赖坤美. 脓毒症并发急性肾损伤的临床预测模型及预后分析[D]. 福建医科大学, 2021.
[4] 王含玉, 罗朋立. 脓毒症合并急性肾损伤的发病机制与最新治疗进展的研究[J]. 临床医学进展, 2023, 13(8): 12362-12368.
[5] 李莹莹, 宋二凯. 脓毒症并发AKI的危险因素及对患者预后水平的影响研究[J]. 青岛医药卫生, 2023, 55(3): 161-165. doi: 10.3969/j.issn.1006-5571.2023.03.001
[6] 白小丽, 敖其, 陈婷婷, 等. 外周血炎症指标在脓毒症肾损伤中的预测价值[J]. 临床急诊杂志, 2024, 25(2): 62-68. doi: 10.13201/j.issn.1009-5918.2024.02.003
[7] 杨建海, 张颖, 张晓强. 血清PCT、hs-CRP及Th17细胞对脓毒症相关急性肾损伤患者行连续肾脏替代治疗法结局的预测价值[J]. 东南大学学报(医学版), 2023, 42(4): 572-577. doi: 10.3969/j.issn.1671-6264.2023.04.013
[8] Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock(Sepsis-3)[J]. Jama, 2016, 315(8): 801-810. doi: 10.1001/jama.2016.0287
[9] Khwaja A. KDIGO clinical practice guidelines for acute kidney injury[J]. Nephron Clin Pract, 2012, 120(4): c179-184. doi: 10.1159/000339789
[10] 娄莹, 马文君, 王子君, 等. 中国高血压临床实践指南计划书[J]. 中华心血管病杂志, 2022, 50(7): 671-675. doi: 10.3760/cma.j.cn112148-20211126-01021
[11] 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4): 315-409.
[12] 中华医学会, 中华医学会杂志社, 中华医学会全科医学分会, 等. 稳定性冠心病基层诊疗指南(2020年)[J]. 中华全科医师杂志, 2021, 20(3): 265-273. doi: 10.3760/cma.j.cn114798-20210120-00079
[13] Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials[J]. Crit Care(London, England), 2019, 23(1): 374. doi: 10.1186/s13054-019-2663-7
[14] Czajka S, Ziębińska K, Marczenko K, et al. Validation of APACHE Ⅱ, APACHE Ⅲ and SAPS Ⅱ scores in in-hospital and one year mortality prediction in a mixed intensive care unit in Poland: a cohort study[J]. BMC Anesthesiol, 2020, 20(1): 296. doi: 10.1186/s12871-020-01203-7
[15] Islam MM, Satici MO, Eroglu SE. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: An extensive literature review[J]. Turk J Emerg Med, 2024, 24(1): 8-19. doi: 10.4103/tjem.tjem_198_23
[16] 尹帅, 陈欣, 徐雁, 等. 全身免疫炎症指数对接受连续性肾脏替代治疗的脓毒症合并急性肾损伤患者短期预测价值[J]. 中华内分泌外科杂志, 2022, 16(3): 356-360. doi: 10.3760/cma.j.cn.115807-20220317-00065
[17] 崔业惠, 李燕, 王芸飞, 等. 全身免疫炎症指数预测脓毒症病死率: 一项回顾性研究[J]. 中华急诊医学杂志, 2024, 33(2): 200-203. doi: 10.3760/cma.j.issn.1671-0282.2024.02.010
[18] Mangalesh S, Dudani S, Malik A. The systemic immune-inflammation index in predicting sepsis mortality[J]. Postgrad Med, 2023, 135(4): 345-351. doi: 10.1080/00325481.2022.2140535
[19] Ma K, Zhang Y, Hao J, et al. Correlation analysis of systemic immune inflammatory index, Serum IL-35 and HMGB-1 with the severity and prognosis of sepsis[J]. Pak J Med Sci, 2023, 39(2): 497-501.
[20] Tang J, Zhong Z, Nijiati M, et al. Systemic inflammation response index as a prognostic factor for patients with sepsis-associated acute kidney injury: a retrospective observational study[J]. J Int Med Res, 2024, 52(3): 3000605241235758. doi: 10.1177/03000605241235758
[21] Ru S, Luo Y. The association and prognostic value of systemic inflammatory response index with short and long-term mortality in patients with sepsis[J]. Medicine, 2023, 102(29): e33967. doi: 10.1097/MD.0000000000033967
[22] 张鼎昊, 尹雪强, 左雷. 脓毒症患者血清IL-1、IL-17的表达水平及其对预后的影响[J]. 广东医学, 2023, 44(9): 1121-1125.
[23] Zhuo L, Chen X, Sun Y, et al. Rapamycin Inhibited Pyroptosis and Reduced the Release of IL-1β and IL-18 in the Septic Response[J]. Biomed Res Int, 2020, 2020: 5960375. doi: 10.1155/2020/5960375
[24] Deng P, Tang N, Li L, et al. Diagnostic value of combined detection of IL-1β, IL-6, and TNF-α for sepsis-induced cardiomyopathy[J]. Med Clin(Barc), 2022, 158(9): 413-417.
[25] Cao J, Liu W, Li Y, et al. Value of IL-1β and IL-23 in Predicting 28-Day Mortality Due to Sepsis: A Retrospective Study[J]. Med Sci Monit, 2023, 29: e940163.
[26] Liu W, Wang X, Wu W. Role and functional mechanisms of IL-17/IL-17R signaling in pancreatic cancer(Review)[J]. Oncol Rep, 2024, 52(5): 144.
[27] 史东剑, 赵大铭, 张坤. 脓毒症相关急性肾损伤患者血清NLRP3、IL-17表达水平与肾功能、肾脏预后的关系[J]. 分子诊断与治疗杂志, 2024, 16(6): 1007-1010, 1014.
[28] 龚志平, 高艳飞, 刘秋霞, 等. 血清IL-33、IL-35对老年脓毒症患者预后的预测价值[J]. 中国老年学杂志, 2023, 43(8): 1859-1861. doi: 10.3969/j.issn.1005-9202.2023.08.019
[29] Ling H, Lin Y, Bao W, et al. Erythropoietin-mediated IL-17 F attenuates sepsis-induced gut microbiota dysbiosis and barrier dysfunction[J]. Biomed Pharmacother, 2023, 165: 115072. doi: 10.1016/j.biopha.2023.115072
[30] Li L, Chen L, Lin F, et al. Study of the Expression of Inflammatory Factors IL-4, IL-6, IL-10, and IL-17 in Liver Failure Complicated by Coagulation Dysfunction and Sepsis[J]. J Inflamm Res, 2021, 14: 1447-1453.
-

计量
- 文章访问数: 202
- 施引文献: 0



 下载:
下载: