Geniposide improves the prognosis of cecal ligation and puncture sepsis model in mice by regulating monocyte phenotype
-
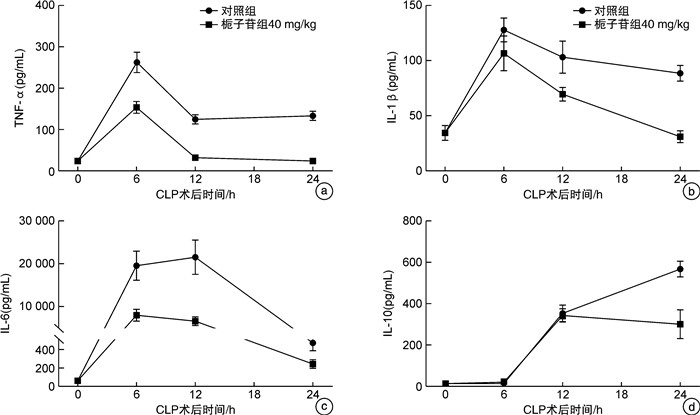
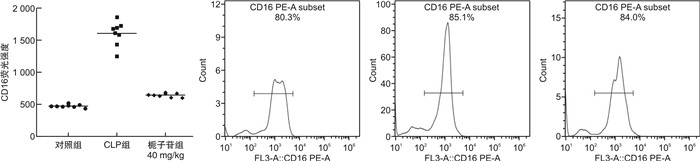
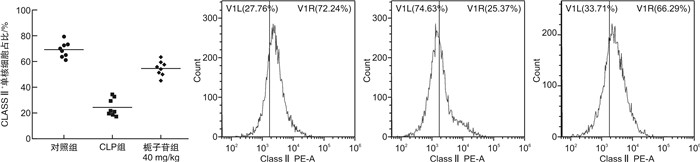
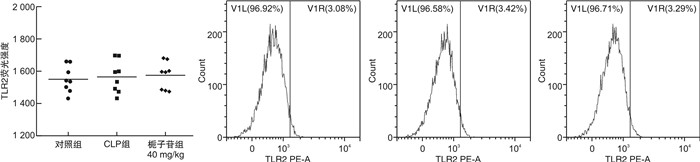
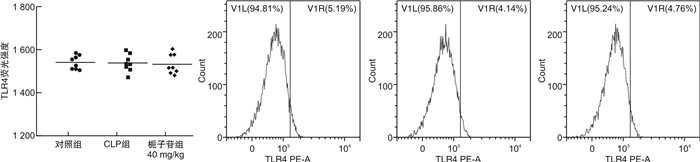
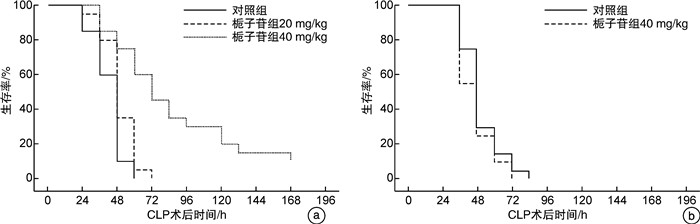
摘要: 目的 探讨不同剂量栀子苷治疗脓毒症的疗效和主要生物学机制。方法 雄性BALB/c小鼠通过盲肠结扎穿孔技术(cecal ligation and puncture,CLP)复制脓毒症模型。在生存实验中,动物被随机分为以下各组,每组20只:于CLP术后0 h、24 h经小鼠尾静脉分别注射栀子苷20 mg/kg、40 mg/kg或生理盐水(对照组);于CLP术后24 h经小鼠尾静脉注射栀子苷40 mg/kg或生理盐水(对照组)。观察不同组别的生存预后;并流式检测单核细胞CD16、MHC-Ⅱ、TLR2、TLR4表达水平;ELISA检测血清TNF-α、IL-1β、IL-6、IL-10浓度;Western Blot测定PPARγ浓度。结果 40 mg/kg栀子苷CLP后0 h和24 h静脉给药能显著改善脓毒症小鼠模型生存预后,小剂量(20 mg/kg)栀子苷和延迟给药(24 h)无显著获益。与对照组相比,有效剂量栀子苷能不同程度地全面抑制脓毒症小鼠血清细胞因子TNF-α、IL-1β、IL-6、IL-10浓度,差异有统计学意义(P < 0.05);能降低脓毒症小鼠24 h单核细胞CD16表达,差异有统计学意义(P < 0.05);能增加脓毒症小鼠24 h单核细胞MHCⅡ表达,差异有统计学意义(P < 0.05);对脓毒症小鼠24 h单核细胞TLR2、TLR4表达无显著影响,差异无统计学意义(P>0.05);能恢复脓毒症小鼠24 h单核细胞PPARγ蛋白活性(0.69±0.02 vs.0.44±0.02),差异有统计学意义(P < 0.05)。结论 早期大剂量栀子苷(40 mg/kg)可以通过调节单核细胞表型,调控细胞因子网络显著改善CLP小鼠脓毒症模型的生存预后。对PPARγ的正性作用可能是栀子苷上游的药理学机制。Abstract: Objective To explore the efficacy and main biological mechanism of geniposide in the treatment of sepsis.Methods Male BALB/c mice replicate a model of septic excess heat through cecal ligation and puncture(CLP). Different doses of geniposide(20 mg/kg, 40 mg/kg) were administered intravenously at 0 h and/or 24 h after CLP. The survival and prognosis of different groups were observed. The expression levels of CD16, MHC-Ⅱ, TLR2, and TLR4 in monocytes were detected by flow cytometry. Serum concentrations of TNF-α, IL-1β, IL-6 and IL-10 were determined by ELISA. The concentrations of PPARγ were determined by Western Blot.Results Intravenous administration of 40 mg/kg geniposide at 0 and 24 hours after CLP significantly improved the survival and prognosis of septic mouse models, while small doses(20 mg/kg) and delayed administration(24 hours) had no significant benefits. Effective dose of geniposide could comprehensively inhibit serum cytokine TNF-α, IL-1 β, IL-6, IL-10 concentration in sepsis mice(P < 0.05). The effective dose of geniposide could reduce the expression of CD16 in monocytes of septic mice at 24 hours(P < 0.05), and increase the expression of MHC Ⅱ in monocytes of septic mice at 24 hours(P < 0.05), but has no significant effect on the expression of TLR2 and TLR4 in monocytes of septic mice at 24 hours(P>0.05). The effective dose of geniposide could restore PPARγ protein activity in monocytes of septic mice at 24 hours(P < 0.05).Conclusion Early high dose Geniposide can significantly improve the prognosis of sepsis by regulating cytokine network, monocyte phenotype. The positive effect on PPARγ may be the upstream pharmacological mechanism of gardeniside.
-
Key words:
- sepsis /
- geniposide /
- cecal ligation and puncture /
- cytokine /
- monocyte /
- PPARγ
-

-
[1] Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock(sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. doi: 10.1001/jama.2016.0287
[2] Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study[J]. Lancet, 2020, 395(10219): 200-211. doi: 10.1016/S0140-6736(19)32989-7
[3] Cohen J. The immunopathogenesis of sepsis[J]. Nature, 2002, 420(6917): 885-891. doi: 10.1038/nature01326
[4] Dellinger RP, Levy MM, Schorr CA, et al. 50 years of sepsis investigation/enlightenment among adults-the long and winding road[J]. Crit Care Med, 2021, 49(10): 1606-1625. doi: 10.1097/CCM.0000000000005203
[5] Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021[J]. Intensive Care Med, 2021, 47(11): 1181-1247. doi: 10.1007/s00134-021-06506-y
[6] Rhee C, Klompas M. Sepsis trends: increasing incidence and decreasing mortality, or changing denominator?[J]. J Thorac Dis, 2020, 12(suppl 1): S89-S100.
[7] Fajgenbaum DC, June CH. Cytokine storm[J]. N Engl J Med, 2020, 383(23): 2255-2273. doi: 10.1056/NEJMra2026131
[8] Stolarski AE, Kim J, Zhang QY, et al. Cytokine drizzle-the rationale for abandoning cytokine storm[J]. Shock, 2021, 56(5): 667-672. doi: 10.1097/SHK.0000000000001769
[9] Kox M, Waalders NJB, Kooistra EJ, et al. Cytokine levels in critically ill patients with COVID-19 and other conditions[J]. JAMA, 2020, 324(15): 1565-1567. doi: 10.1001/jama.2020.17052
[10] van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis[J]. Immunity, 2021, 54(11): 2450-2464. doi: 10.1016/j.immuni.2021.10.012
[11] Guan J, Wang Z, Liu X, et al. IL-6 and IL-10 closely correlate with bacterial bloodstream infection[J]. Iran J Immunol, 2020, 17(3): 185-203.
[12] Pinheiro da Silva F, Aloulou M, Skurnik D, et al. CD16 promotesEscherichia colisepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation[J]. Nat Med, 2007, 13(11): 1368-1374. doi: 10.1038/nm1665
[13] Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RⅢ(CD16) deficient mice[J]. Immunity, 1996, 5(2): 181-188. doi: 10.1016/S1074-7613(00)80494-X
[14] Monneret G, Venet F, Pachot A, et al. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony[J]. Mol Med, 2008, 14(1-2): 64-78. doi: 10.2119/2007-00102.Monneret
[15] Brunialti MK, Martins PS, Barbosa de Carvalho H, et al. TLR2, TLR4, CD14, CD11B, and CD11C expressions on monocytes surface and cytokine production in patients with sepsis, severe sepsis, and septic shock[J]. Shock, 2006, 25(4): 351-357. doi: 10.1097/01.shk.0000217815.57727.29
[16] Martins PS, Brunialti MK, Martos LS, et al. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis[J]. Crit Care, 2008, 12(1): R25. doi: 10.1186/cc6801
-




 下载:
下载: