-
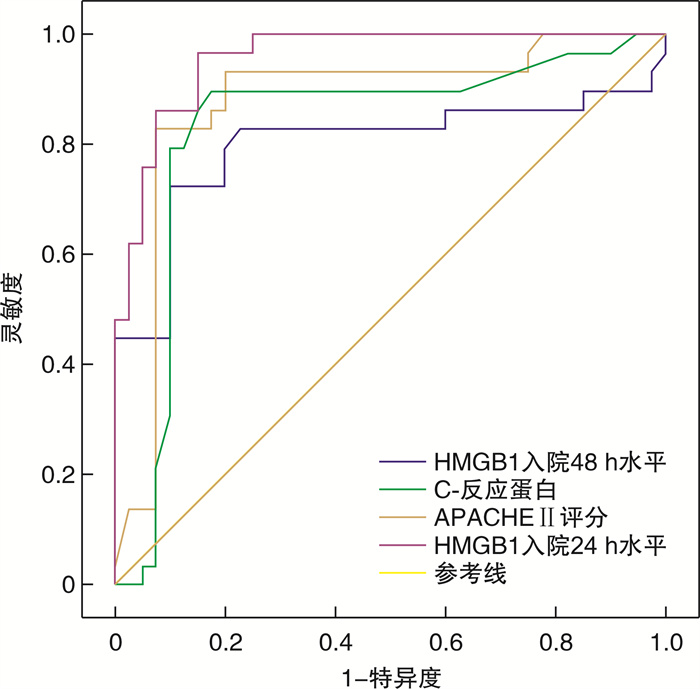
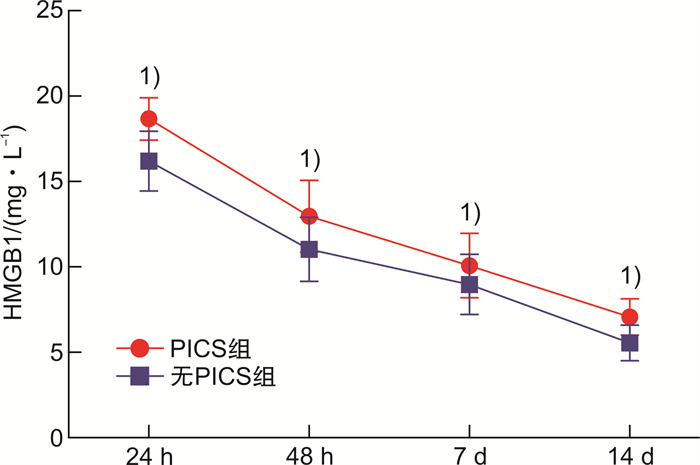
摘要: 目的 探讨血清高迁移率族蛋白B1(HMGB1)预测重症急性胰腺炎(SAP)患者发生持续炎症反应-免疫抑制-分解代谢综合征(PICS)的价值。方法 选取2018年1月—2020年12月内蒙古自治区人民医院收治的SAP患者141例,根据是否发生PICS将患者分为PICS组(n=39)和无PICS组(n=102)。酶联免疫吸附试验检测入院24 h、48 h、7 d、14 d时血清HMGB1表达水平。PICS影响因素分析采用logistic多因素分析;采用受试者工作特征曲线(ROC)分析HMGB1预测PICS的价值,计算曲线下面积(AUC)、灵敏度和特异度。结果 PICS组患者入院24 h、48 h、7 d、14 d时血清HMGB1表达水平均高于无PICS组(P < 0.05)。logistic多因素分析结果显示,APACHE Ⅱ评分、C-反应蛋白水平、HMGB1入院24 h水平、HMGB1入院48 h水平为SAP患者发生PICS的独立影响因素(P < 0.05)。HMGB1入院24 h水平预测SAP患者发生PICS的AUC为0.940,灵敏度为92.05%、特异度为91.47%;HMGB1入院48 h水平预测SAP患者发生PICS的AUC为0.785,灵敏度为80.07%、特异度为84.00%。结论 早期检测血清HMGB1水平有助于预测SAP患者发生PICS。
-
关键词:
- 血清 /
- 高迁移率族蛋白B1 /
- 重症急性胰腺炎 /
- 持续炎症反应-免疫抑制-分解代谢综合征 /
- 预测
Abstract: Objective To investigate the value of serum high mobility group box B1(HMGB1) in predicting persistent inflammatory response-immunosuppression-catabolic syndrome(PICS) in patients with severe acute pancreatitis(SAP).Methods A total of 141 SAP patients admitted to our hospital from January 2018 to December 2020 were selected, and the patients were divided into PICS group(n=39) and none-PICS group(n=102) according to whether PICS occurred. The expression level of serum HMGB1 was detected by ELISA at 24 h, 48 h, 7 d and 14 d after admission. Logistic multivariate analysis was used to analyze the influencing factors of PICS; receiver operating characteristic curve(ROC) was used to analyze the value of HMGB1 in predicting PICS, and the area under the curve(AUC), sensitivity and specificity were calculated.Results The expression levels of serum HMGB1 in the PICS group were higher than those in the non-PICS group at 24 h, 48 h, 7 d, and 14 d after admission(P < 0.05). Logistic multivariate analysis showed that APACHE Ⅱ score, CRP level, HMGB1 level at 24 hours after admission, and HMGB1 level at 48 hours after admission were the independent influencing factors of PICS in SAP patients(P < 0.05). The AUC of HMGB1 level at 24 hours after admission was 0.940, the sensitivity was 92.05%, and the specificity was 91.47%; the AUC of HMGB1 level at 48 hours after admission was 0.785, the sensitivity was 80.07%, and the specificity was 84.00%.Conclusion Early detection of serum HMGB1 level is helpful to predict PICS in SAP patients. -

-
表 1 2组患者临床资料比较
指标 PICS组(n=39) 无PICS组(n=102) P 年龄/岁 46.01±6.34 45.11±5.87 >0.05 性别(男/女) 21/18 62/40 >0.05 病因/例 >0.05 胆源性 19 50 高脂血症性 14 41 其他 6 11 APACHE Ⅱ评分/分 12.09±1.87 10.56±1.76 < 0.05 Balthazar CT评分/分 3.45±1.12 3.08±0.76 < 0.05 C-反应蛋白/(mg·L-1) 167.90±64.05 132.45±53.12 < 0.05 白细胞计数/(×109·L-1) 13.87±4.01 13.03±3.82 >0.05 淋巴细胞计数/(×109·L-1) 0.76±0.28 0.83±0.31 >0.05 白蛋白/(g·L-1) 32.09±5.08 33.25±6.02 >0.05 ICU住院时间/d 21.78±4.01 17.34±1.19 < 0.05 总住院时间/d 65.09±10.23 27.12±4.05 < 0.05 脓毒性休克/例 15 14 < 0.05 多器官功能衰竭/例 4 3 < 0.05 死亡/例 5 3 < 0.05 表 2 2组血清HMGB1表达水平比较
mg/L,X±S 指标 PICS组(n=39) 无PICS组(n=102) P HMGB1 24 h 18.67±1.24 16.20±1.75 < 0.05 48 h 12.97±2.10 11.03±1.87 < 0.05 7 d 10.08±1.89 8.97±1.76 < 0.05 14 d 7.07±1.06 5.55±0.74 < 0.05 C反应蛋白 24 h 167.90±64.05 132.45±53.12 < 0.05 48 h 123.34±36.12 110.23±38.23 < 0.05 7 d 36.89±10.01 24.89±8.23 < 0.05 14 d 23.67±6.87 14.78±4.70 < 0.05 表 3 PICS发生的logistic多因素分析
因素 OR 95%CI P APACHE Ⅱ评分 1.581 1.002~1.897 < 0.05 C-反应蛋白 1.123 1.021~1.907 < 0.05 HMGB1入院24 h水平 1.897 1.210~2.765 < 0.05 HMGB1入院48 h水平 1.209 1.008~1.786 < 0.05 表 4 各指标预测PICS的效能分析
指标 AUC 95%CI 灵敏度/% 特异度/% HMGB1入院24 h水平 0.940 0.918~0.998 92.05 91.47 APACHE Ⅱ评分 0.853 0.781~0.971 85.93 84.03 C-反应蛋白 0.821 0.782~0.934 82.96 87.20 HMGB1入院48 h水平 0.785 0.668~0.945 80.07 84.00 -
[1] 方琦, 陶京, 常剑. 重症急性胰腺炎持续炎症-免疫抑制-分解代谢综合征的诊断与治疗研究进展[J]. 中华消化外科杂志, 2019, 18(7): 701-704. doi: 10.3760/cma.j.issn.1673-9752.2019.07.016
[2] Mankowski RT, Anton SD, Ghita GL, et al. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression, and catabolism syndrome(PICS)after sepsis[J]. J Gerontol A Biol Sci Med Sci, 2021, 77(1): 188-196.
[3] Zhao JJ, Sun TL, Wu SD, et al. High mobility group box 1: an immune-regulatory protein[J]. Curr Gene Ther, 2019, 19(2): 100-109. doi: 10.2174/1566523219666190621111604
[4] Yang H, Wang HC, Andersson U. Targeting inflammation driven by HMGB1[J]. Front Immunol, 2020, 11((10)): 484.
[5] Li L, Lu YQ. The regulatory role of high-mobility group protein 1 in sepsis-related immunity[J]. Front Immunol, 2021, 11: 601815. doi: 10.3389/fimmu.2020.601815
[6] 中华医学会消化病学分会胰腺疾病学组, 中华胰腺病杂志编辑委员会, 中华消化杂志编辑委员会. 中国急性胰腺炎诊治指南(2019年, 沈阳)[J]. 中华消化杂志, 2019, 39(11): 721-730. doi: 10.3760/cma.j.issn.0254-1432.2019.11.001
[7] Bahtouee M, Eghbali SS, Maleki N, et al. Acute Physiology and Chronic Health Evaluation Ⅱ score for the assessment of mortality prediction in the intensive care unit: a single-centre study from Iran[J]. Nurs Crit Care, 2019, 24(6): 375-380. doi: 10.1111/nicc.12401
[8] Taydas O, Unal E, Karaosmanoglu AD, et al. Accuracy of early CT findings for predicting disease course in patients with acute pancreatitis[J]. Jpn J Radiol, 2018, 36(2): 151-158. doi: 10.1007/s11604-017-0709-9
[9] Komara NL, Paragomi P, Greer PJ, et al. Severe acute pancreatitis: capillary permeability model linking systemic inflammation to multiorgan failure[J]. Am J Physiol Gastrointest Liver Physiol, 2020, 319(5): G573-G583. doi: 10.1152/ajpgi.00285.2020
[10] Sendler M, van den Brandt C, Glaubitz J, et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis[J]. Gastroenterology, 2020, 158(1): 253-269. e14. doi: 10.1053/j.gastro.2019.09.040
[11] Mira JC, Brakenridge SC, Moldawer LL, et al. Persistent inflammation, immunosuppression and catabolism syndrome[J]. Crit Care Clin, 2017, 33(2): 245-258. doi: 10.1016/j.ccc.2016.12.001
[12] Darden DB, Brakenridge SC, Efron PA, et al. Biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome(PICS)in chronic critical illness(CCI)after surgical sepsis[J]. Ann Surg, 2021, 274(4): 664-673.
[13] Zhong M, Pan TT, Sun NN, et al. Early prediction for persistent inflammation-immunosuppression catabolism syndrome in surgical sepsis patients[J]. Int J Gen Med, 2021, 14: 5441-5448. doi: 10.2147/IJGM.S331411
[14] Yang N, Li BQ, Ye B, et al. The long-term quality of life in patients with persistent inflammation-immunosuppression and catabolism syndrome after severe acute pancreatitis: a retrospective cohort study[J]. J Crit Care, 2017, 42: 101-106. doi: 10.1016/j.jcrc.2017.07.013
[15] Downes KJ, Barreto EF. Estimating renal function for drug dosing in critically ill patients with persistent inflammation, immunosuppression and catabolism syndrome[J]. Intern Emerg Med, 2021, 16(7): 1751-1753. doi: 10.1007/s11739-021-02770-4
[16] Zhang JL, Luo WC, Miao CH, et al. Hypercatabolism and anti-catabolic therapies in the persistent inflammation, immunosuppression, and catabolism syndrome[J]. Front Nutr, 2022, 9: 941097. doi: 10.3389/fnut.2022.941097
[17] 刘东全, 胡金龙, 李敏, 等. 简易PICS评分在评价急性胰腺炎严重程度中的价值[J]. 重庆医学, 2021, 50(11): 1852-1856, 1861. doi: 10.3969/j.issn.1671-8348.2021.11.012
[18] Yang H, Andersson U, Brines M. Neurons are a primary driver of inflammation via release of HMGB1[J]. Cells, 2021, 10(10): 2791. doi: 10.3390/cells10102791
[19] Lee JH, Song WJ, An JH, et al. Role of serum high-motility group box-1(HMGB1) concentration as a prognostic factor in canine acute pancreatitis: a pilot study[J]. Res Vet Sci, 2021, 141: 26-32. doi: 10.1016/j.rvsc.2021.09.013
[20] Gao YZ, Wang LM, Niu ZQ, et al. miR-340-5p inhibits pancreatic acinar cell inflammation and apoptosis via targeted inhibition of HMGB1[J]. Exp Ther Med, 2022, 23(2): 140.
[21] Ye LS, Zhang Q, Cheng YS, et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+regulatory B cell expansion[J]. J Immunother Cancer, 2018, 6(1): 145. doi: 10.1186/s40425-018-0451-6
[22] Watanabe H, Son M. The immune tolerance role of the HMGB1-RAGE axis[J]. Cells, 2021, 10(3): 564. doi: 10.3390/cells10030564
[23] 苏兆亮, 张永健, 倪萍, 等. 重组高迁移率族蛋白1(rHMGB1)体外诱导小鼠骨髓细胞向髓源性抑制性细胞(MDSC)分化[J]. 细胞与分子免疫学杂志, 2016, 32(10): 1362-1365, 1371. https://www.cnki.com.cn/Article/CJFDTOTAL-XBFM201610014.htm
-

| 引用本文: | 李彩霞, 孟文勤, 郝颖楠, 等. HMGB1早期预测重症急性胰腺炎患者发生PICS的价值研究[J]. 临床急诊杂志, 2022, 23(12): 859-862. doi: 10.13201/j.issn.1009-5918.2022.12.011 |
| Citation: | LI Caixia, MENG Wenqin, HAO Yingnan, et al. Value of HMGB1 in early prediction of PICS in severe acute pancreatitis patients[J]. J Clin Emerg, 2022, 23(12): 859-862. doi: 10.13201/j.issn.1009-5918.2022.12.011 |
- Figure 1.
- Figure 2.



 下载:
下载: