A correlation analysis of the the expression of NLRP3 inflammatory body in peripheral blood mononuclear cells with patient prognosis with cardiac arrest
-
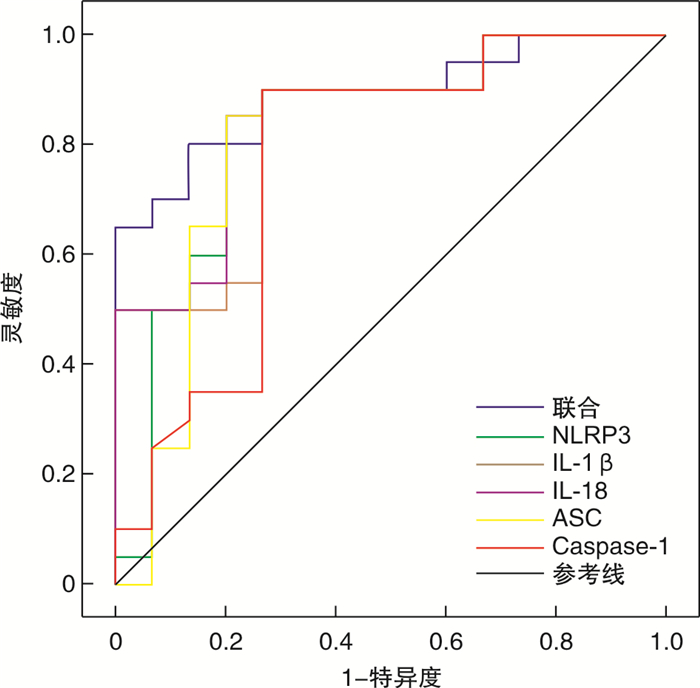
摘要: 目的 探究外周血单核细胞NLRP3炎症小体表达与心搏骤停后患者预后的相关性。方法 选取2014年6月—2021年6月因心搏骤停于我院急诊科收治并恢复自主循环的120例患者(病例组),根据患者是否存活出院,分为存活组(35例)及死亡组(85例)。同时收集同期在我院体检的80例健康志愿者为对照组。收集受试者人口学信息、心搏骤停与复苏的相关信息及恢复自主循环后12 h时外周血单核细胞NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA及Caspase-1 mRNA表达水平。6个月后对存活组患者进行随访,评估其神经系统及心血管系统预后情况。采用受试者工作特征曲线分析NLRP3炎症小体表达在患者预后评估中的价值。结果 与对照组相比,病例组外周血单核细胞NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA及Caspase-1 mRNA表达水平显著升高(P< 0.05)。与存活组相比,死亡组外周血单核细胞NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA及Caspase-1 mRNA表达水平显著升高(P< 0.05)。在预后方面,外周血单核细胞NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA、Caspase-1 mRNA表达升高与不良神经系统及心血管系统预后相关(P< 0.05)。ROC曲线显示,外周血单核细胞NLRP3、IL-1β、IL-18、ASC和Caspase-1联合评估心搏骤停患者预后的ROC曲线下面积为0.890(95%CI:0.835~0.945),此时灵敏度为80.0%,特异度为86.7%。结论 在心搏骤停患者中,外周血单核细胞NLRP3炎症小体表达水平明显升高,且表达水平升高与心搏骤停患者院内死亡相关。NLRP3炎症小体表达与心搏骤停存活患者长期心血管系统及神经系统预后相关,且对患者长期心血管系统预后具有一定预测价值。
-
关键词:
- 心搏骤停 /
- 核苷酸结合寡聚化结构域样受体蛋白3 /
- 白细胞介素-1β /
- 白细胞介素-18
Abstract: Objective To investigate the correlation between the expression of NLRP3 inflammatory bodies in peripheral blood monocytes and the prognosis of patients with cardiac arrest.Methods One hundred and twenty patients with cardiac arrest admitted to our hospital from June, 2014 to June, 2021 were selected as patient group. During the same period 80 healthy participants were selected as control group. The demographic information, comorbidity, cardiac arrest information, cardiopulmonary resuscitation information and laboratory findings(NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA and Caspase-1 mRNA) were collected. Patient group was divided into survival group(n=35) and death group(n=85) according to whether the patients survived to discharge. Follow up the patients in the survival group 6 months later to assess the neurological and cardiovascular outcomes. Receiver operating characteristic curve(ROC) was applied to analyze the effect of NLRP3 inflammatory body on the prognosis of patient with cardiac arrest.Results Compared with the control group, the expression of NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA and Caspase-1 mRNA were statistically significantly evaluated in the patient group(P< 0.05). Moreover, compared with the survival group, the expression of NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA and Caspase-1 mRNA were statistically significantly evaluated in the death group(P< 0.05). Correlation existed between the increase of NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA and Caspase-1 mRNA and the neurological and cardiovascular prognosis(P< 0.05). The ROC curve showed that the AUC of combination diagnosis in prediction of the prognosis of cardiac arrest was 0.890(95%CI: 0.835-0.945). The sensitivity and specificity was 80.0% and 86.7% respectively.Conclusion In patients with cardiac arrest, the expression of NLRP3 inflammasome in peripheral blood monocytes was significantly increased, and increased expression level was associated with nosocomial death in patients with cardiac arrest. NLRP3 inflammasome expression is associated with long-term neurological and cardiovascular prognosis in cardiac arrest survivors, and has predictive value for long-term cardiovascular prognosis. -

-
表 1 200例受试者基线数据资料
例(%) 基线资料 对照组(80例) 存活组(35例) 死亡组(85例) P 年龄/岁 68.8±5.7 66.5±5.8 70.8±6.9 0.002 性别 0.154 男 45(56.3) 18(51.4) 57(67.1) 女 35(43.8) 17(48.6) 28(32.9) 慢性病史 高血压 31(38.8) 15(42.9) 48(56.5) 0.359 糖尿病 34(42.5) 18(51.4) 55(64.7) 0.305 心血管系统疾病a) 31(38.8) 18(51.4) 43(50.6) 0.172 心搏骤停原因 0.707 心源性 21(60.0) 48(56.5) 呼吸源性 7(20.0) 24(28.2) 神经源性 1(2.9) 4(4.7) 其他b) 6(17.1) 9(10.6) 心搏骤停相关目击者 31(88.6) 70(82.4) 0.398 旁观者心肺复苏 19(54.3) 53(62.4) 0.414 心搏骤停地点 0.683 家中 22(62.9) 50(58.8) 其他 13(37.1) 35(41.2) 入院脑功能表现分级 < 0.001 1级 21(60.0) 3(3.5) 2级 10(28.6) 6(7.1) 3~5级 4(11.4) 76(89.4) 注:a)心血管系统疾病包括冠心病、心肌病、风湿性心脏病; b)其他心搏骤停原因包括溺水、有机磷中毒、电击等。 表 2 3组受试者NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA、Caspase-1 mRNA水平比较
X±S 指标 对照组(80例) 存活组(35例) 死亡组(85例) P NLRP3 mRNA 0.886±0.217 1.235±0.302 1.761±0.492 < 0.001 IL-1β mRNA 0.978±0.199 1.222±0.215 1.793±0.402 < 0.001 IL-18 mRNA 1.024±0.196 1.412±0.321 1.732±0.476 < 0.001 ASC mRNA 0.924±0.215 1.295±0.221 1.507±0.199 < 0.001 Caspase-1 mRNA 0.990±0.198 1.305±0.158 1.780±0.203 < 0.001 表 3 长期随访事件组与非事件组NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA、Caspase-1 mRNA水平比较
X±S 指标 事件组(20例) 非事件组(15例) P NLRP3 mRNA 1.384±0.228 1.036±0.277 < 0.001 IL-1β mRNA 1.325±0.199 1.086±0.157 0.001 IL-18 mRNA 1.575±0.261 1.194±0.263 < 0.001 ASC mRNA 1.391±0.142 1.166±0.246 0.002 Caspase-1 mRNA 1.367±0.127 1.223±0.162 0.006 表 4 长期随访神经功能良好、轻度受损、重度受损组NLRP3 mRNA、IL-1β mRNA、IL-18 mRNA、ASC mRNA、Caspase-1 mRNA水平比较
X±S 指标 神经功能良好(21例) 轻度神经功能受损(10例) 重度神经功能受损(4例) P NLRP3 mRNA 1.126±0.284 1.409±0.162 1.646±0.104 0.001 IL-1β mRNA 1.146±0.195 1.326±0.093 1.553±0.213 0.001 IL-18 mRNA 1.310±0.294 1.493±0.066 2.013±0.229 < 0.001 ASC mRNA 1.223±0.218 1.417±0.128 1.542±0.112 0.008 Caspase-1 mRNA 1.225±0.114 1.446±0.136 1.575±0.075 < 0.001 -
[1] 朱天威. 基于死亡证明的心脏性猝死流行情况分析[D]; 广州中医药大学, 2020.
[2] Dalessio L. Post-Cardiac Arrest Syndrome[J]. AACN Adv Crit Care, 2020, 31(4): 383-393. doi: 10.4037/aacnacc2020535
[3] Gando S, Wada T. Disseminated intravascular coagulation in cardiac arrest and resuscitation[J]. J Thromb Haemost, 2019, 17(8): 1205-1216. doi: 10.1111/jth.14480
[4] Sunde K, Pytte M, Jacobsen D. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest[J]. Resuscitation, 2007, 73(1): 29-39. doi: 10.1016/j.resuscitation.2006.08.016
[5] Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis[J]. Intensive Care Med, 2021, 47(12): 1393-1414. doi: 10.1007/s00134-021-06548-2
[6] Czerwińska-Jelonkiewicz K, Grand J, Tavazzi G. Acute respiratory failure and inflammatory response after out-of-hospital cardiac arrest: results of the Post-Cardiac Arrest Syndrome(PCAS)pilot study[J]. Eur Heart J Acute Cardiovasc Care, 2020, 9(4_suppl): S110-s121. doi: 10.1177/2048872619895126
[7] Bro-Jeppesen J, Kjaergaard J, Stammet P. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 ℃ or 36 ℃[J]. Resuscitation, 2016, 98: 1-8. doi: 10.1016/j.resuscitation.2015.10.009
[8] 李超, 吴允孚, 刘军. 中性粒细胞/淋巴细胞、血小板/淋巴细胞预测心肺复苏术自主循环恢复患者预后的价值[J]. 徐州医科大学学报, 2019, 39(12): 893-898. doi: 10.3969/j.issn.2096-3882.2019.12.09
[9] Toldo S, Mezzaroma E, Buckley L F. Targeting the NLRP3 inflammasome in cardiovascular diseases[J]. Pharmacol Ther, 2021, 236: 108053.
[10] Mangan M S J, Olhava EJ, Roush W R. Targeting the NLRP3 inflammasome in inflammatory diseases[J]. Nat Rev Drug Discov, 2018, 17(8): 588-606. doi: 10.1038/nrd.2018.97
[11] Suceveanu AI, Mazilu L, Katsiki N. NLRP3 Inflammasome Biomarker-Could Be the New Tool for Improved Cardiometabolic Syndrome Outcome[J]. Metabolites, 2020, 10(11): 839-844.
[12] Krishnan SM, Sobey CG, Latz E. IL-1β and IL-18: inflammatory markers or mediators of hypertension?[J]. Br J Pharmacol, 2014, 171(24): 5589-602. doi: 10.1111/bph.12876
[13] Panchal A R, Bartos JA, Cabañas J G. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care[J]. Circulation, 2020, 142(16_suppl_2): S366-S468.
[14] Jennett B, Bond M. Assessment of outcome after severe brain damage[J]. Lancet, 1975, 1(7905): 480-484.
[15] Mentzelopoulos SD, Zakynthinos S G. Post-cardiac arrest syndrome: pathological processes, biomarkers and vasopressor support, and potential therapeutic targets[J]. Resuscitation, 2017, 121: A12-A14. doi: 10.1016/j.resuscitation.2017.10.013
[16] Reis C, Akyol O, Araujo C. Pathophysiology and the Monitoring Methodsfor Cardiac Arrest Associated Brain Injury[J]. Int J Mol Sci, 2017, 18(1): 19-23
[17] Adrie C, Adib-Conquy M, Laurent I. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome[J]. Circulation, 2002, 106(5): 562-568. doi: 10.1161/01.CIR.0000023891.80661.AD
[18] Wang Y, Liu X, Shi H. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases[J]. Clin Transl Med, 2020, 10(1): 91-106. doi: 10.1002/ctm2.13
[19] Adams JA. Endothelium and cardiopulmonary resuscitation[J]. Crit Care Med, 2006, 34(12 Suppl): S458-S465.
[20] Zhang C, Brandon NR, Koper K. Invasion of Peripheral Immune Cells into Brain Parenchyma after Cardiac Arrest and Resuscitation[J]. Aging Dis, 2018, 9(3): 412-425. doi: 10.14336/AD.2017.0926
[21] Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy?[J]. Acta Neuropathol, 2019, 137(5): 693-714. doi: 10.1007/s00401-018-1930-z
[22] Su XL, Wang SH, Komal S. The caspase-1 inhibitor VX765 upregulates connexin 43 expression and improves cell-cell communication after myocardial infarction via suppressing the IL-1β/p38 MAPK pathway[J]. Acta Pharmacol Sin, 2022.
[23] Wu S, Li Z, Ye M. VX765, a Specific Caspase-1 Inhibitor, Alleviates Lung Ischemia Reperfusion Injury by Suppressing Endothelial Pyroptosis and Barrier Dysfunction[J]. Biomed Res Int, 2021, 2021: 4525988.
[24] Audia JP, Yang XM, Crockett ES. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y(12) receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function[J]. Basic research in cardiology, 2018, 113(5): 32. doi: 10.1007/s00395-018-0692-z
[25] Peberdy MA, Andersen L W, Abbate A. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study[J]. Resuscitation, 2016, 103: 117-124. doi: 10.1016/j.resuscitation.2016.01.006
[26] 白颐, 田慈, 翟樯榕. 心脏骤停后患者外周血细胞变化与预后的相关性[J]. 中国急救医学, 2021, 41(5): 380-385. doi: 10.3969/j.issn.1002-1949.2021.05.003
[27] 张丽冉, 李艳华, 夏瑞雪. 急性脑梗死患者血清OPN、NLRP3水平与神经损伤和预后的关系[J]. 脑与神经疾病杂志, 2021, 29(8): 502-506. https://www.cnki.com.cn/Article/CJFDTOTAL-LYSJ202108010.htm
[28] Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 Inflammasome Over-activation Increases Lethality of SARS-CoV-2 Pneumonia in Elderly Patients[J]. Aging Dis, 2020, 11(4): 756-762. doi: 10.14336/AD.2020.0601
-




 下载:
下载: