Clinical value of serum inflammatory marker ST2 in predicting the severity and prognosis of acute pancreatitis
-
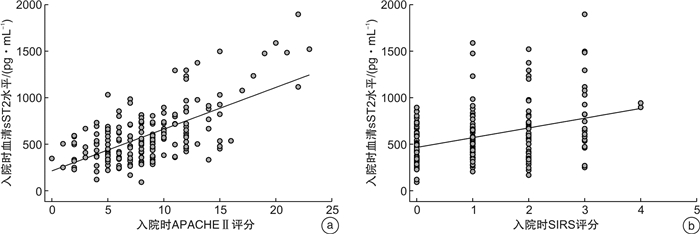
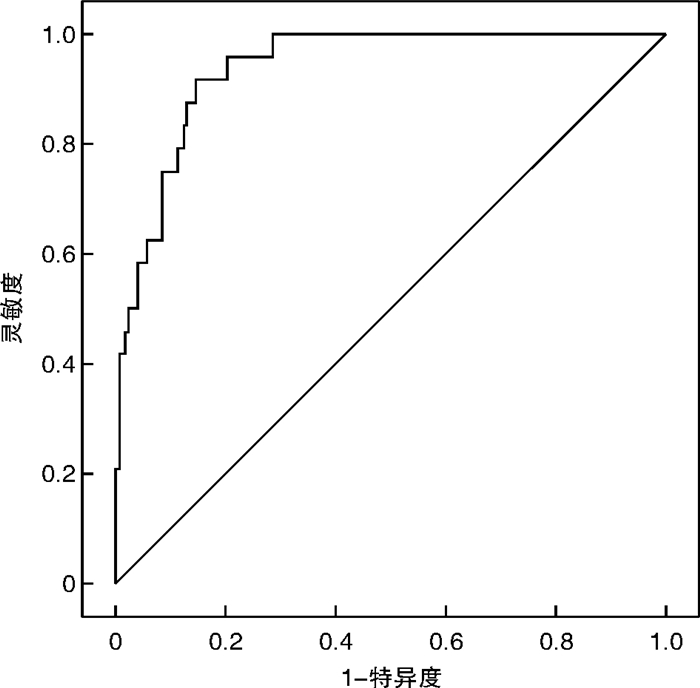
摘要: 目的 探讨血清炎症标志物可溶性生长刺激表达基因2蛋白(sST2)与急性胰腺炎(AP)严重程度及预后的关系。方法 选取2018年1月—2020年11月在首都医科大学附属北京世纪坛医院诊治的203例AP患者(AP组),根据改良的Marshall评分进行器官功能衰竭评估,按AP严重程度分为轻度AP(MAP组)、中度AP(MSAP组)和重度AP(SAP组)。另选取100例我院健康体检者作为对照组。采用酶联免疫吸附法检测入院时、入院48 h后和缓解期(出院前24 h)血清sST2水平。结果 与对照组相比,AP患者血清干扰素(IFN-γ)、白细胞介素-2(IL-2)、IL-4、IL-13、IL-33、sST2水平均升高,差异有统计学意义(P< 0.05)。然而只有血清sST2水平与疾病严重程度呈正相关(P< 0.05)。入院时血清sST2水平预测SAP的受试者工作特征曲线下面积为0.940(95%CI: 0.904~0.976)。血清sST2水平和急性生理与慢性健康评分Ⅱ(APACHE Ⅱ)、全身炎症反应综合征评分呈正相关(P< 0.001)。经单因素和多因素logistic回归分析,入院时血清sST2>526.0 pg/mL是预测患者院内死亡的独立危险因素(P< 0.05)。对于幸存的AP患者,与入院时相比,入院48 h、缓解期时血清sST2水平逐渐降低(P< 0.001)。结论 血清sST2有可能成为AP诊断和监测疾病严重程度的候选生物标志物,同时也是患者院内死亡的独立预测因子。
-
关键词:
- 急性胰腺炎 /
- 可溶性生长刺激表达基因2蛋白 /
- 严重程度 /
- 预后
Abstract: Objective To investigate the relationship between serum inflammatory marker soluble growth stimulation expression gene 2 protein(sST2) and severity and prognosis of acute pancreatitis(AP).Methods A total of 203 patients with AP treated in our hospital from January 2018 to November 2020 were selected. According to the improved Marshall score, they were divided into mild AP(MAP) group, moderate AP(MSAP) group and severe AP(SAP) group. A healthy control group of 100 patients were selected from local hospital staff. Serum sST2 levels were detected by enzyme-linked immunosorbent assay at admission, 48 hours after admission and in remission(24 hours before discharge).Results Compared with healthy control group, serum levels of interferon-γ, interleukin-2(IL-2), IL-4, IL-13, IL-33 and sST2 were increased in AP patients(P< 0.05). However, only serum sST2 levels were positively correlated with disease severity(P< 0.05). The area under the receiver operating characteristic curve for predicting SAP by serum sST2 level at admission was 0.940(95%CI: 0.904-0.976). There was positive correlation between serum sST2 level and acute physiology and chronic health score Ⅱ, systemic inflammatory response syndrome score(P< 0.001). Univariate and multivariate logistic regression analysis showed that admission serum ST2>526.0 pg/mL was an independent risk factor for predicting in-hospital death(P< 0.05). For the surviving AP patients, the level of serum sST2 decreased gradually at 48 hours of admission and remission compared with that at admission(P< 0.001).Conclusion sST2 may be a candidate biomarker for AP diagnosis and monitoring of disease severity, and it is also an independent predictor of in-hospital death. -

-
表 1 AP组和对照组血清炎症因子水平比较
pg/mL,M(P25,P75) 血清炎症因子 对照组(100例) AP组(203例) Z P IFN-γ 0.75(0.27,1.41) 1.50(0.90,2.20) -2.895 0.017 IL-2 0.71(0.46,1.39) 1.07(0.70,1.68) -2.491 0.038 IL-4 0.42(0.23,1.24) 0.99(0.43,2.42) -3.158 0.005 IL-13 0.30(0.09,0.75) 0.87(0.69,1.18) -3.251 0.001 IL-33 1.85(0.67,4.33) 7.34(5.71,9.85) -5.179 < 0.001 sST2 117.85(68.91,189.65) 526.0(374.45,700.85) -8.923 < 0.001 表 2 AP患者一般资料及临床病理特征比较
例(%),M(P25,P75) 特征 MAP组(117例) MSAP组(62例) SAP组(24例) χ2/F P 年龄/岁 77.00(66.25,85.25) 73.00(52.75,81.25) 64.00(46.00,78.00) 0.790 0.674 男性 70(59.8) 39(62.9) 14(58.3) 0.219 0.897 BMI 22.68±4.30 22.76±3.89 23.83±4.15 0.777 0.461 病因学 10.227 0.596 胆结石 48(41.0) 20(32.2) 7(29.1) 过度饮酒 22(18.8) 16(25.8) 7(29.1) 特发性 36(30.7) 21(33.8) 8(33.3) 药源性 1(0.8) 1(1.6) 1(4.1) 胰肠吻合口狭窄 6(5.1) 0 1(4.1) 癌症 1(0.8) 2(3.2) 0 其他 3(2.5) 2(3.2) 0 肝硬化 2(1.71) 23(37.1)1) 12(50.0)1) 52.481 < 0.001 急性胰周积液 62(52.9) 31(50.0) 9(37.5) 1.914 0.384 炎症扩展至直肠膀胱 13(11.1) 9(14.5) 0 3.794 0.150 发病至入院时间/h 11.50(4.38,21.88) 15.75(5.63,25.88) 7.75(2.63,22.50) 1.580 0.454 Charlson合并症指数/分 0.50(0.00,2.00) 1.00(0.00,2.00) 0.00(0.00,0.75) 3.225 0.199 APACHE Ⅱ/分 9.00(6.25,10.00) 9.00(6.25,13.75) 12.00(8.25,16.50)1)2) 22.585 < 0.001 SIRS评分/分 1.00(0.00,1.00) 1.00(0.00,2.00) 2.50(2.00,3.00)1)2) 36.319 < 0.001 体温峰值/℃ 36.81±0.83 36.97±0.84 36.77±0.78 0.898 0.409 脉搏/(次·min-1) 85.00(77.25,98.50) 77.00(67.25,94.75) 71.50(61.00,89.00) 4.396 0.111 收缩压/mmHg 134.59±28.06 135.66±27.44 133.79±26.82 0.049 0.952 白细胞计数/(×104·μL-1) 11139.32±4855.80 11524.19±4052.67 10737.50±4915.88 0.281 0.755 血小板计数/(×104·μL-1) 21.72±7.64 21.41±4.22 22.80±6.97 0.376 0.687 CRP峰值/(mg·dL-1) 0.54(0.22,3.02) 0.98(0.07,4.61) 0.68(0.18,2.20) 0.094 0.954 淀粉酶峰值/(U·L-1) 1296.00(603.50,1563.75) 1764.50(748.50,3129.25) 1026.50(260.50,2527.75) 0.056 0.972 乳酸脱氢酶峰值/(U·L-1) 314.50(244.75,395.00) 305.00(224.75,447.00) 258.00(220.75,420.25) 0.721 0.697 尿素氮峰值/(mg·dL-1) 16.35(12.13,24.18) 19.60(13.93,34.05) 14.75(10.85,19.20) 2.515 0.284 血肌酐峰值/(mg·dL-1) 0.75(0.55,0.83) 0.88(0.68,1.91) 0.72(0.65,0.87) 1.112 0.573 血钙峰值/(mg·dL-1) 9.23±0.62 9.02±0.51 9.13±0.32 2.741 0.067 白蛋白峰值/(g·L-1) 37.94±5.45 37.92±5.34 41.40±4.321)2) 12.835 < 0.001 总胆红素峰值/(μmol·L-1) 1.10(0.83,2.03) 1.85(0.88,2.58) 1.70(1.13,2.38) 2.981 0.225 血清炎症因子 IFN-γ/(pg·mL-1) 1.10(0.83,1.78) 1.70(1.13,2.50) 1.75(1.20,2.38) 2.463 0.292 IL-2/(pg·mL-1) 1.03(0.53,1.63) 1.14(0.69,1.57) 1.27(0.74,2.23) 2.230 0.328 IL-4/(pg·mL-1) 0.82(0.71,0.97) 0.83(0.63,1.35) 0.85(0.16,3.93) 0.786 0.675 IL-13/(pg·mL-1) 0.80(0.59,1.15) 0.76(0.58,1.10) 0.86(0.71,1.19) 0.186 0.911 IL-33/(pg·mL-1) 6.71(5.60,7.88) 7.78(6.06,10.03) 9.72(6.97,11.72) 5.654 0.059 sST2/(pg·mL-1) 492.45(360.68,602.13) 589.85(458.90,748.08) 987.20(761.50,1355.25) 58.855 < 0.001 注:1 mmHg=0.133 kPa。与MAP组相比,1)P < 0.05; 与MSAP组相比,2)P < 0.05。 表 3 单因素和多因素logistic回归分析入院血清sST2与院内死亡的关系
血清sST2/(pg·mL-1) 模型1 模型2 模型3 HR(95%CI) P HR(95%CI) P HR(95%CI) P < 374.45 1.000 - 1.000 - 1.000 - 374.45~526.0 1.514(1.032~3.997) 0.019 1.019(1.001~3.457) 0.033 0.917(0.572~3.450) 0.413 >526.0~700.85 3.126(1.986~9.286) < 0.001 1.971(1.224~6.850) 0.001 1.422(1.079~3.564) 0.007 >700.85 9.009(4.005~21.014) < 0.001 4.375(2.019~11.423) < 0.001 4.120(1.753~10.662) < 0.001 注:模型1:单因素分析; 模型2:校正年龄、性别、BMI、Charlson合并症指数、发病至入院时间; 模型3:在模型2的基础上进一步校正体温、SBP、WBC、PLT以及其他实验室指标。 -
[1] 李素青, 王利军. 老年急性胰腺炎患者血清胆红素和白蛋白与病情严重程度及死亡风险的相关性[J]. 中国老年学杂志, 2021, 41(19): 4224-4227. doi: 10.3969/j.issn.1005-9202.2021.19.021
[2] 邓弘扬, 魏丰贤, 张宇浩, 等. 新型血清学指标早期预测急性胰腺炎严重程度及预后的研究进展[J]. 中国免疫学杂志, 2021, 37(10): 1274-1278. doi: 10.3969/j.issn.1000-484X.2021.10.025
[3] Zhang Y, Cheng B, Wu ZW, et al. Serum soluble suppression of tumorigenicity 2 as a novel inflammatory marker predicts the severity of acute pancreatitis[J]. World J Gastroenterol, 2021, 27(38): 6489-6500. doi: 10.3748/wjg.v27.i38.6489
[4] 赵小龙, 陈文亮. lL-33/ST2信号通路在急性胰腺炎及纤维化中的作用[J]. 中国现代普通外科进展, 2019, 22(2): 121-125. https://www.cnki.com.cn/Article/CJFDTOTAL-PWJZ201902010.htm
[5] Pascual-Figal DA, Bayes-Genis A, Asensio-Lopez MC, et al. The Interleukin-1 Axis and Risk of Death in Patients With Acutely Decompensated Heart Failure[J]. J Am Coll Cardiol, 2019, 73(9): 1016-1025.
[6] Aimo A, Januzzi JL Jr, Bayes-Genis A, et al. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week[J]. J Am Coll Cardiol, 2019, 74(17): 2193-2203. doi: 10.1016/j.jacc.2019.08.1039
[7] Homsak E, Ekart R. ST2 as a novel prognostic marker in end-stage renal disease patients on hemodiafiltration[J]. Clin Chim Acta, 2018, 477: 105-112. doi: 10.1016/j.cca.2017.12.006
[8] Shieh JM, Tseng HY, Jung F, et al. Elevation of IL-6 and IL-33 Levels in Serum Associated with Lung Fibrosis and Skeletal Muscle Wasting in a Bleomycin-Induced Lung Injury Mouse Model[J]. Mediators Inflamm, 2019, 2019: 7947596.
[9] Billiar IM, Guardado J, Abdul-Malak O, et al. Elevations in Circulating sST2Levels Are Associated With In-Hospital Mortality and Adverse Clinical Outcomes After Blunt Trauma[J]. J Surg Res, 2019, 244: 23-33. doi: 10.1016/j.jss.2019.05.057
[10] Magro F, Lopes S, Silva M, et al. Soluble human Suppression of Tumorigenicity 2 is associated with endoscopic activity in patients with moderate-to-severe ulcerative colitis treated with golimumab[J]. Therap Adv Gastroenterol, 2019, 12: 1756284819869141.
[11] 中华医学会外科学分会胰腺外科学组. 中国急性胰腺炎诊治指南(2021)[J]. 中华消化外科杂志, 2021, 20(7): 730-739. doi: 10.3760/cma.j.cn115610-20210622-00297
[12] 陈文亮, 段俊芳, 白露露, 等. NF-κB、IL-33及sST2在急性胰腺炎合并急性肾损伤中的价值研究[J]. 中国现代普通外科进展, 2018, 21(10): 778-782. https://www.cnki.com.cn/Article/CJFDTOTAL-PWJZ201810007.htm
[13] Mahmutovic Persson I, Menzel M, Ramu S, et al. IL-1β mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation[J]. Respir Res, 2018, 19(1): 16. doi: 10.1186/s12931-018-0725-z
[14] Kurimoto M, Watanabe T, Kamata K, et al. IL-33 as a Critical Cytokine for Inflammation and Fibrosis in Inflammatory Bowel Diseases and Pancreatitis[J]. Front Physiol, 2021, 12: 781012. doi: 10.3389/fphys.2021.781012
[15] Rodriguez-Nicolas A, Martínez-Chamorro A, Jiménez P, et al. TH1 and TH2 Cytokine Profiles as Predictors of Severity in Acute Pancreatitis[J]. Pancreas, 2018, 47(4): 400-405. doi: 10.1097/MPA.0000000000001006
[16] 隗世波, 刘青云, 钟群琼. IL-33/ST2信号通路在急性呼吸窘迫综合征患者中的表达变化及意义研究[J]. 临床急诊杂志, 2019, 20(4): 302-306, 311. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC201904011.htm
[17] 齐晓瑜, 李敬, 张羽, 等. 可溶性生长刺激表达基因2蛋白和MHR及NLR在重症心力衰竭中的相关性研究[J]. 临床急诊杂志, 2022, 23(4): 283-288. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC202204013.htm
[18] Rezar R, Paar V, Seelmaier C, et al. Soluble suppression of tumorigenicity 2 as outcome predictor after cardiopulmonary resuscitation: an observational prospective study[J]. Sci Rep, 2021, 11(1): 21756. doi: 10.1038/s41598-021-01389-x
[19] Alladina J, Levy SD, Cho JL, et al. Plasma Soluble Suppression of Tumorigenicity-2 Associates with Ventilator Liberation in Acute Hypoxemic Respiratory Failure[J]. Am J Respir Crit Care Med, 2021, 203(10): 1257-1265. doi: 10.1164/rccm.202005-1951OC
[20] Ip C, Luk KS, Yuen VLC, et al. Soluble suppression of tumorigenicity 2(sST2) for predicting disease severity or mortality outcomes in cardiovascular diseases: A systematic review and meta-analysis[J]. Int J Cardiol Heart Vasc, 2021, 37: 100887.
[21] 张棱, 谷阳. 血浆SST2和NGAL水平对急性ST段抬高型心肌梗死患者预后的评估价值[J]. 临床急诊杂志, 2020, 21(9): 692-695. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZLC202009003.htm
[22] 孔夏, 张新超. 血清可溶性ST2对脓毒症预后的判断价值研究[J]. 中华急诊医学杂志, 2018, 27(4): 394-398.
-




 下载:
下载: