-
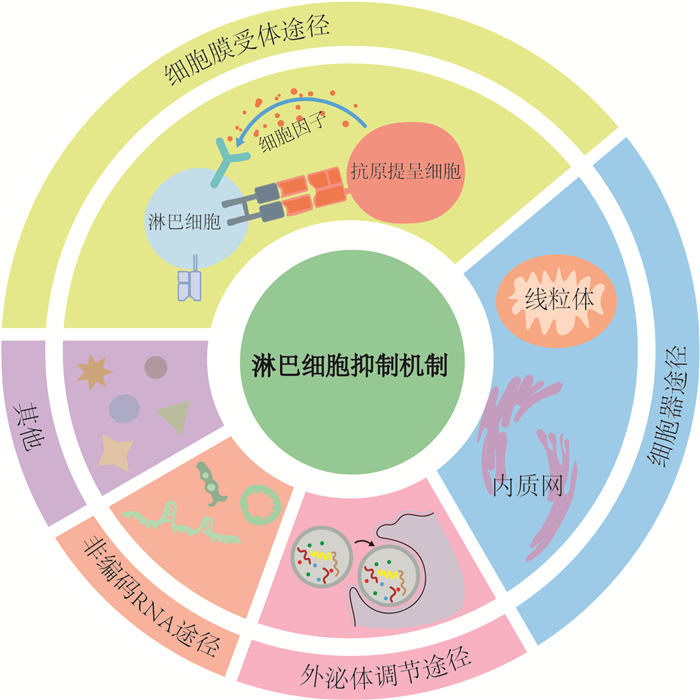
摘要: 脓毒症是指由宿主对感染反应失调所致危及生命的器官功能障碍综合征,其病理机制复杂,最重要的特征是免疫功能紊乱,几乎所有免疫细胞均参与其中。淋巴细胞作为人体重要的免疫细胞,在脓毒症的发生发展和转归中均起着十分重要的作用,近年来对其机制及应用的研究也越来越深入,已成为重要的脓毒症早期筛查、预后评估、免疫监测和调理治疗点。本文旨在通过综述脓毒症中淋巴细胞抑制机制及其研究进展,包括膜受体途径,如负性共刺激分子、细胞因子等,细胞器途径,如线粒体、内质网等,外泌体途径和非编码RNA途径等,以及金属离子介导的铁死亡、铜死亡等,以期为其机制的进一步探索和脓毒症的免疫调理治疗研究提供重要参考。Abstract: Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Its pathological mechanism is complex, and the most important feature is immune dysfunction, in which almost all immune cells are involved. As an important group of immune cells in our human body, lymphocytes play a very important role in the occurrence, development and outcome of sepsis. In recent years, the research on its mechanism and application has become more and more in-depth, and it has become an important point for early screening, prognosis assessment, immune monitoring and conditioning treatment of sepsis. The aim of this article is to review the mechanisms of lymphocyte suppression in sepsis, including membrane receptor pathways, such as negative costimulatory molecules and cytokines, organelle pathways, such as mitochondria and endoplasmic reticulum, exosome pathways, non-coding RNA pathways, and metal ion-mediated ferroptosis, copper death, etc. This study provides an important reference for the further exploration of its mechanism and the research of immunomodulatory therapy for sepsis.
-
Key words:
- sepsis /
- lymphocyte /
- negative costimulatory molecules /
- non-coding RNA /
- exosome
-

-
[1] Liu D, Huang SY, Sun JH, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options[J]. Mil Med Res, 2022, 9(1): 56.
[2] Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study[J]. Lancet, 2020, 395(10219): 200-211. doi: 10.1016/S0140-6736(19)32989-7
[3] Liu YC, Yao Y, Yu MM, et al. Frequency and mortality of sepsis and septic shock in China: a systematic review and meta-analysis[J]. BMC Infect Dis, 2022, 22(1): 564. doi: 10.1186/s12879-022-07543-8
[4] Weng L, Xu Y, Yin P, et al. National incidence and mortality of hospitalized sepsis in China[J]. Crit Care, 2023, 27(1): 84. doi: 10.1186/s13054-023-04385-x
[5] 张萌, 杨正飞. 脓毒症中T细胞亚群变化诱导的免疫抑制[J]. 临床急诊杂志, 2024, 25(3): 153-158. https://lcjz.whuhzzs.com/article/doi/10.13201/j.issn.1009-5918.2024.03.011
[6] Roe K. NK-cell exhaustion, B-cell exhaustion and T-cell exhaustion-the differences and similarities[J]. Immunology, 2022, 166(2): 155-168. doi: 10.1111/imm.13464
[7] Wang ZB, Zhang WZ, Chen LL, et al. Lymphopenia in sepsis: a narrative review[J]. Crit Care, 2024, 28(1): 315. doi: 10.1186/s13054-024-05099-4
[8] Wen XY, Xie B, Yuan SY, et al. The "self-sacrifice" of Immune Cells in sepsis[J]. Front Immunol, 2022, 13: 833479. doi: 10.3389/fimmu.2022.833479
[9] Zheng XT, Chen WW, Gong FC, et al. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: a review[J]. Front Immunol, 2021, 12: 711939. doi: 10.3389/fimmu.2021.711939
[10] Yan HF, Zou T, Tuo QZ, et al. Ferroptosis: mechanisms and links with diseases[J]. Signal Transduct Target Ther, 2021, 6: 49. doi: 10.1038/s41392-020-00428-9
[11] Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins[J]. Science, 2022, 375(6586): 1254-1261. doi: 10.1126/science.abf0529
[12] Wakeley ME, Gray CC, Monaghan SF, et al. Check point inhibitors and their role in immunosuppression in sepsis[J]. Crit Care Clin, 2020, 36(1): 69-88. doi: 10.1016/j.ccc.2019.08.006
[13] Wu H, Tang TX, Deng H, et al. Immune checkpoint molecule Tim-3 promotes NKT cell apoptosis and predicts poorer prognosis in Sepsis[J]. Clin Immunol, 2023, 254: 109249. doi: 10.1016/j.clim.2023.109249
[14] Huang SY, Liu D, Sun JH, et al. Tim-3 regulates sepsis-induced immunosuppression by inhibiting the NF-κB signaling pathway in CD4 T cells[J]. Mol Ther, 2022, 30(3): 1227-1238. doi: 10.1016/j.ymthe.2021.12.013
[15] Sun YN, Ding RY, Chang YK, et al. Immune checkpoint molecule TIGIT manipulates T cell dysfunction in septic patients[J]. Int Immunopharmacol, 2021, 101(Pt B): 108205.
[16] de Lima MHF, Hiroki CH, de Fátima Borges V, et al. Sepsis-induced immunosuppression is marked by an expansion of a highly suppressive repertoire of FOXP3+ T-regulatory cells expressing TIGIT[J]. J Infect Dis, 2022, 225(3): 531-541. doi: 10.1093/infdis/jiab405
[17] Tao TZ, Bo LL, Li T, et al. High-affinity anti-VISTA antibody protects against sepsis by inhibition of T lymphocyte apoptosis and suppression of the inflammatory response[J]. Mediators Inflamm, 2021, 2021: 6650329.
[18] Gray CC, Biron-Girard B, Wakeley ME, et al. Negative immune checkpoint protein, VISTA, regulates the CD4+ treg population during sepsis progression to promote acute sepsis recovery and survival[J]. Front Immunol, 2022, 13: 861670. doi: 10.3389/fimmu.2022.861670
[19] Deng Z, Zheng Y, Cai P, et al. The role of B and T lymphocyte attenuator in respiratory system diseases[J]. Front Immunol, 2021, 12: 635623. doi: 10.3389/fimmu.2021.635623
[20] Wang Q, Deng J, Sun JH, et al. PDGFR kinase inhibitor protects against septic death via regulation of BTLA[J]. Sci China Life Sci, 2022, 65(10): 1917-1928. doi: 10.1007/s11427-021-2136-y
[21] 孙燃, 黄佳敏, 刘璐, 等. 脓毒症小鼠中性粒细胞通过CD80/CTLA-4途径影响T淋巴细胞功能[J]. 中华危重病急救医学, 2021, 33(7): 849-854.
[22] Lei XL, Zhao GY, Xie YW, et al. mTOR deletion alleviates CD4+ T-cell dysfunction in sepsis through reducing CTLA4 accumulation mediated by rescuing autophagy[J]. Mediators Inflamm, 2024, 2024: 4233439. doi: 10.1155/2024/4233439
[23] Gaborit BJ, Roquilly A, Louvet C, et al. Regulatory T cells expressing tumor necrosis factor receptor type 2 play a major role in CD4+ T-cell impairment during sepsis[J]. J Infect Dis, 2020, 222(7): 1222-1234. doi: 10.1093/infdis/jiaa225
[24] Coman O, Grigorescu BL, Huţanu A, et al. The role of programmed cell death 1/programmed death ligand 1(PD-1/PD-L1) axis in sepsis-induced apoptosis[J]. Medicina, 2024, 60(7): 1174. doi: 10.3390/medicina60071174
[25] Liu JL, Song K, Lin BQ, et al. HMGB1 promotes neutrophil PD-L1 expression through TLR2 and mediates T cell apoptosis leading to immunosuppression in sepsis[J]. Int Immunopharmacol, 2024, 133: 112130. doi: 10.1016/j.intimp.2024.112130
[26] Patel AG, Moxham S, Bamezai AK. Ly-6A-induced growth inhibition and cell death in a transformed CD4+ T cell line: role of tumor necrosis factor-Α[J]. Arch Immunol Ther Exp, 2023, 71(1): 4. doi: 10.1007/s00005-023-00670-3
[27] Lambert K, Diggins KE, Jones BE, et al. IL-6-Driven pSTAT1 response is linked to T cell features implicated in early immune dysregulation[J]. Front Immunol, 2022, 13: 935394. doi: 10.3389/fimmu.2022.935394
[28] Bernstein ZJ, Shenoy A, Chen A, et al. Engineering the IL-4/IL-13 axis for targeted immune modulation[J]. Immunol Rev, 2023, 320(1): 29-57. doi: 10.1111/imr.13230
[29] Iamartino L, Brandi ML. The calcium-sensing receptor in inflammation: recent updates[J]. Front Physiol, 2022, 13: 1059369. doi: 10.3389/fphys.2022.1059369
[30] Huang L, Zhang XD, Fan JY, et al. EGFR promotes the apoptosis of CD4+ T lymphocytes through TBK1/Glut1 induced Warburg effect in sepsis[J]. J Adv Res, 2023, 44: 39-51. doi: 10.1016/j.jare.2022.04.010
[31] Durand M, Hagimont E, Louis H, et al. The β1-adrenergic receptor contributes to sepsis-induced immunosuppression through modulation of regulatory T-cell inhibitory function[J]. Crit Care Med, 2022, 50(9): e707-e718. doi: 10.1097/CCM.0000000000005503
[32] Albayati S, Vemulapalli H, Tsygankov AY, et al. P2Y12 antagonism results in altered interactions between platelets and regulatory T cells during sepsis[J]. J Leukoc Biol, 2021, 110(1): 141-153. doi: 10.1002/JLB.3A0220-097R
[33] Harrington JS, Ryter SW, Plataki M, et al. Mitochondria in health, disease, and aging[J]. Physiol Rev, 2023, 103(4): 2349-2422. doi: 10.1152/physrev.00058.2021
[34] Nedel W, Deutschendorf C, Portela LVC. Sepsis-induced mitochondrial dysfunction: a narrative review[J]. World J Crit Care Med, 2023, 12(3): 139-152. doi: 10.5492/wjccm.v12.i3.139
[35] Bai GX, Wang H, Cui N. mTOR pathway mediates endoplasmic reticulum stress-induced CD4+ T cell apoptosis in septic mice[J]. Apoptosis, 2022, 27(9): 740-750.
[36] Lu G, Li QQ, Liu JJ, et al. Inhibition of endoplasmic reticulum stress and the downstream pathways protects CD4+ T cells against apoptosis and immune dysregulation in sepsis[J]. IUBMB Life, 2022, 74(11): 1070-1080. doi: 10.1002/iub.2666
[37] Murao A, Brenner M, Aziz M, et al. Exosomes in sepsis[J]. Front Immunol, 2020, 11: 2140. doi: 10.3389/fimmu.2020.02140
[38] Liu Y, Luo TT, Li H, et al. Protective effect of endothelial progenitor cell-derived exosomal microRNA-382-3p on sepsis-induced organ damage and immune suppression in mice[J]. Am J Transl Res, 2022, 14(10): 6856-6873.
[39] Gao K, Jin JM, Huang CY, et al. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines[J]. Front Immunol, 2019, 10: 1560. doi: 10.3389/fimmu.2019.01560
[40] Deng JN, Li YQ, Liu Y, et al. Exosomes derived from plasma of septic patients inhibit apoptosis of T lymphocytes by down-regulating bad via hsa-miR-7-5p[J]. Biochem Biophys Res Commun, 2019, 513(4): 958-966. doi: 10.1016/j.bbrc.2019.04.051
[41] 邹琪, 赵士兵, 吴强, 等. 脓毒症患者外周血淋巴细胞中微小RNA-126表达量与细胞凋亡及预后的相关性分析[J]. 中华危重病急救医学, 2020, 32(8): 938-942.
[42] Liu D, Xie LX. The mechanism of miRNSAs on regulating lymphocyte proliferation and apoptosis in sepsis[J]. Chest, 2016, 149(4): A180. doi: 10.1016/j.chest.2016.02.186
[43] Chen JX, Xu X, Zhang S. Silence of long noncoding RNA NEAT1 exerts suppressive effects on immunity during sepsis by promoting microRNA-125-dependent MCEMP1 downregulation[J]. IUBMB Life, 2019, 71(7): 956-968. doi: 10.1002/iub.2033
[44] Kumar D, Gurrapu S, Wang Y, et al. LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of incipient metastatic cells[J]. Nat Cancer, 2024, 5(2): 262-282. doi: 10.1038/s43018-023-00695-9
[45] Lai Y, Gao FF, Ge RT, et al. Metal ions overloading and cell death[J]. Cell Biol Toxicol, 2024, 40(1): 72. doi: 10.1007/s10565-024-09910-4
[46] Dennhardt S, Ceanga IA, Baumbach P, et al. Cell-free DNA in patients with sepsis: long term trajectory and association with 28-day mortality and sepsis-associated acute kidney injury[J]. Front Immunol, 2024, 15: 1382003. doi: 10.3389/fimmu.2024.1382003
[47] Chen DY, Li K, Pan LH, et al. TCF7 and LEF-1 downregulation in sepsis promotes immune suppression by inhibiting CD4+ T cell proliferation[J]. Microb Pathog, 2023, 184: 106362. doi: 10.1016/j.micpath.2023.106362
-

计量
- 文章访问数: 133
- 施引文献: 0



 下载:
下载: